Which One of the Following Is a Pure Substance
It is found in its pure form in milk wheat and many fruits. A pure substance usually participates in a chemical reaction to form predictable products.

Distinguishing Between Mixtures And Pure Substances Chemistry Study Com
Which one of the following is a pure substance.

. V passing of electric current through water and the water breaking down into hydrogen and oxygen gases. Vi dissolving common salt in water. I and ii B.
Liquid oxygen question C. It is not a pure substance. Mixture 2the following substances are examples of compound EXCEPT.
Correct option is A Aerated water is a homogeneous mixture of carbon dioxide and water. It has a constant composition. Salt and water C.
100 2 ratings Transcribed image text. Which of the following is a pure substance. Milk Question 2 Which of the following is a chemical property of propane gas.
A pure substance produces chemical reaction to form expected products. Extensive intensive Which separation method is better suited for separating. 1which one of the following is a pure substance.
H2O2 is the pure substance of the group. Alcohol and water B. Seawater is a mixture of bunches of things.
Math NEED HELP ASAP. These substances mainly have a constant or uniform composition throughout. Iv boiling of water to form steam.
Steak O iced tea elemental hydrogen urine dust The symbol for the element sodium is OHg ОMe Sn Na Pb When we say that lead is a denser metal than aluminum are we talking about an extensive or intensive property. Pure substances include elements and compounds. Chemistry questions and answers.
Its free of any sort of contaminants. Which of the following is a pure substance Asodium BMilk Cblood Dsaline solution Estainless steel. Which one of the following is a pure substance.
In addition to this a pure substance which is made up of gold consists of only particles of gold. Classify the following as chemical or physical changes. I pure substance contain only one kind of particles ii pure substance may be compounds or mixtures ii pure substance have the same composition throughout iv pure substances can be exemplified by all elements other than nickel A.
Gold Au is found in its pure form in nature. A pure substance is different from a - geneous mixture because a pure substance has only one component and can not be easily separated by any physical means. Milk is a heterogeneous mixture of fat which is a form of ester and sugar a form of carbohydrate and hence is not a pure substance like an element or a compound.
A recipe of a fruit punch calls for 2 cups of orange juice to 1 cup of apple juice. They have same boiling and melting point. 2 Which of the following is a pure substance.
Carbon dioxide is a compound while oxygen and zinc are elements which are a type of pure substance. Table sugar 3Which of the following does NOT form a solution. Question 1 Which one of the following is a pure substance not a mixture.
Which one of the following is a pure substance. After leaving a liquid to settle it does not seperate. Salt is a pure substance as it has the same chemical composition N aCl throughout.
I cutting of trees. Iii rusting of almirah. 1point Chocolate milk Orange juice Seawater Hydrogen peroxide H2O2 Math.
Which one of the following is a pure substance. They have fixed boiling and melting point. It is not a pure substance while carbon dioxide is a compound zinc and oxygen are elements which are a type of pure substance.
It is sold as 3 30 90 H2O2 with different percentages between. The constituent particles of a pure substance are the same in their chemical nature. Iron filings and water D.
Multiple samples of the unknown substance melt at the same temperature. Substances like lemonade muddy water and soft drinks are mixtures. Answer saved Points out of 100 Select one.
For example is a molecule and it is a pure substance. Which one of the following is a pure substance. Oct 3 2013.
The usual diluent is H2O. Also substances that contain two or more different element which are chemically combined in a fixed ratio by mass are known as compounds. 835 students attemted this question.
How much orange juice and how much apple juice is needed to make 12 cups of this fruit punch. After leaving a liquid to settle it separates. Which of the the following statements are true for pure substance.
A pure substance consists of only a single type of particle. Steel is a homogeneous mixture however it is made from iron and carbon. They are also pure substances.
It possesses resilient properties throughout the sample. Tap water rock air elemental oxygen apple. Ii melting of butter in a pan.
Aerated drinks contain carbon dioxide gas as solute and water liquid as solvent. Which of the following is good evidence that an unknown sample is a pure substance. A pure substance is defined as the substance that is made up of only one type of atom or only one type of molecule.
Technically however one cant buy PURE H2O2. Pulpy orange juice Flag O b. Pure substances are mostly homogeneous in nature containing only one type of atoms or molecules.
The substances have fixed boiling and melting points. Substances which are commonly found in pure forms include water table salt brass bronze diamond gold and saline solution. However the mixture have different types of substances in different ratio.
Multiple samples of the unknown substance melt.

Solved Question 9 Which One Of The Following Is A Pure Chegg Com
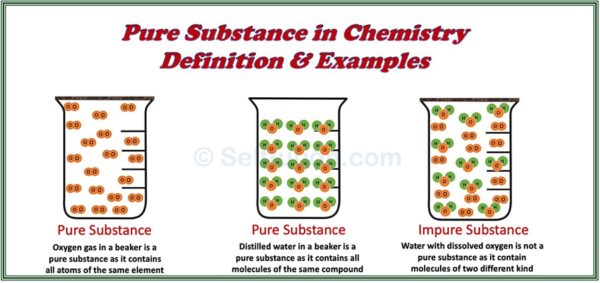
Pure Substance In Chemistry Definition And Examples Selftution

Solved Question 15 Which One Of The Following Is A Pure Chegg Com

Chem 1 Introduction 1 Flashcards Quizlet

Warm Up Define These Terms Mixtures Elements Compounds Heterogeneous Ppt Video Online Download

File Mixtures And Pure Substances 2x2 Svg Wikipedia
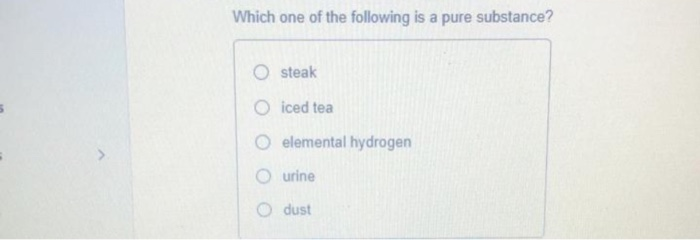
Solved Which One Of The Following Is A Pure Substance Steak Chegg Com

What Are The Types Of Pure Substances Compounds Elements Videos

Pure Substances And Mixtures Diagram Educational Chemistry For Kids Cartoon Style Vector Ill Pure Products Elements Compounds And Mixtures Chemistry For Kids

Classification Of Matter Chemistrygod

Who Wants To Pass Science 9 Ppt Download

Chemistry The Central Science Chapter 1 Section 2
Pure Substances And Mixtures Neds Declassified
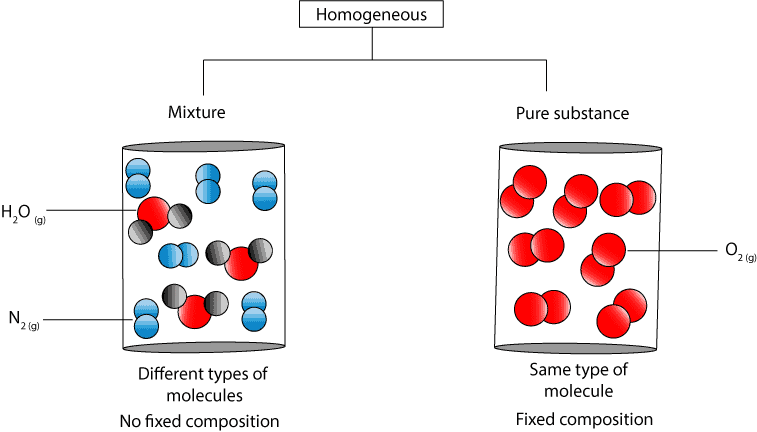
If A Substance Is Homogeneous Is It A Pure Substance

Solved Which One Of The Following Is A Pure Substance Milk Chegg Com

What Are The Types Of Pure Substances And Mixtures A Plus Topper
Pure Substances And Mixtures Neds Declassified
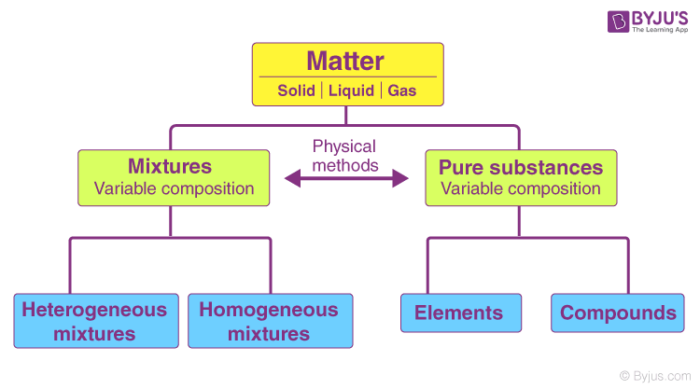
What Is Pure Substance Definition Examples Difference Between Pure Substance Mixture

Comments
Post a Comment